Drive Stronger Business Outcomes with
OPAL
Fit-for-purpose global content delivers…
- Stronger Engagement
- Higher Conversion Rates
- Increased Revenues
- Reduced Risk
To recognize these business outcomes, you need a Service Delivery Platform supported by state-of-the-art AI technologies, configurable workflows and expert talent. You need OPAL.

Improved Customer Engagement

Every day, through localized content, you have the opportunity to improve customer engagement. Better engagement is proven to drive stronger business outcomes.
Recognize benefits that are:
Fast
Accelerates time to market
Profitable
Delivers business outcomes with tangible KPIs
Efficient
Drives cost savings with advanced automation
Easy
Democratizes translations
Comprehensive
Supports multiple departments and content use cases
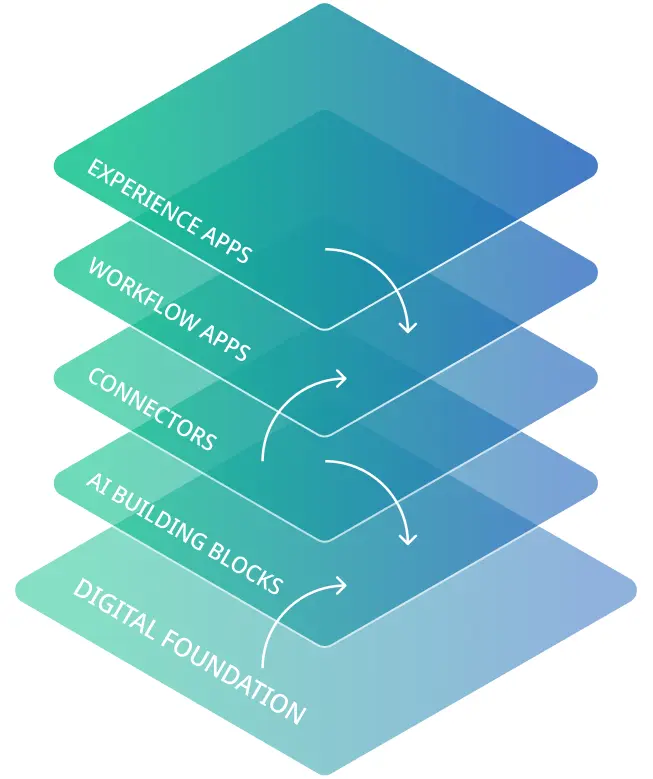
OPAL – An AI-enabled Service Delivery Platform to amplify your global content.
OPAL is built on a digital foundation informed by 25+ years of enterprise language services leadership. OPAL uses machine translation engines, large language models, and natural language processing technology to rapidly automate translations at scale; fit-for-purpose workflows to streamline processing; and experts-in-the-loop to ensure the highest level of quality when your content use cases demand it.
CONFIGURABLE
MODULAR
FIT-FOR-PURPOSE
CONFIGURABLE
MODULAR
FIT-FOR-PURPOSE
CONFIGURABLE
Service Delivery Platform
Empower your content creators to get content translated quickly from within the native work surfaces they use every day—with OPAL’s experience apps.
Benefit from fit-for-purpose workflow apps that govern the translation, review and approval processes related to specific content types and industries.
Accelerate your time to value with OPAL’s pre-built connectors to your enterprise content systems.
Future-proof your global content strategy with modular AI building blocks that leverage the world’s most advanced foundational models.
Capitalize on OPAL’s digital foundation that includes data pipes, intelligent rule engines and AI-enabled agents.
Enlist the help of OPAL’s global expert community for your highest value content.
Get Started With OPal
Explore how Welocalize supports your use cases
Welocalize’s solutions are purpose-built to address the specific content types and use cases within your business.
- Marketing Localization
- Website Localization
- Transcreation
- Multilingual SEO
- Patent Translation & Filing
- Cross-Border Litigation Translation
- General Corporate Matters
- Compliance Translation
- Product Localization
- Software Localization
- Testing
- Voiceover (Human & AI-Generated)
- E-Learning Localization
- Video Localization & Translation
- Linguistic Validation
- Pharmacovigilance
- Clinical Translation
- Instructions for Use
- Labeling
- Regulatory Documentation
- LLM Model Evaluation, Selection & Training
- MT Model Training
- AI Ecosystem Optimization
- Generative AI & LLMs
- Relevance & Intent
- Data Annotation & Natural Language Solutions
- Data Generation
- Bespoke Workforce
- Computer Vision
Trusted by leading global enterprises
Welocalize is a trusted partner to some of the world’s leading companies. Explore some of their stories.







“You know the saying, ‘Suppliers will give you what you ask for, but partners will give you what you need?’ That’s a very important distinction. That’s the environment we have created with Welocalize. It’s been a very successful partnership for many years.”
Director, Global Translation Team – DELL
Technology News & Insights

AI in Action: Combining People, Processes, and Technology
We live in an AI-driven content transformation era that is…

CASE STUDY: Revolutionizing Marketing Localization for Global Tech Giant
This case study explores how a leading global technology company…